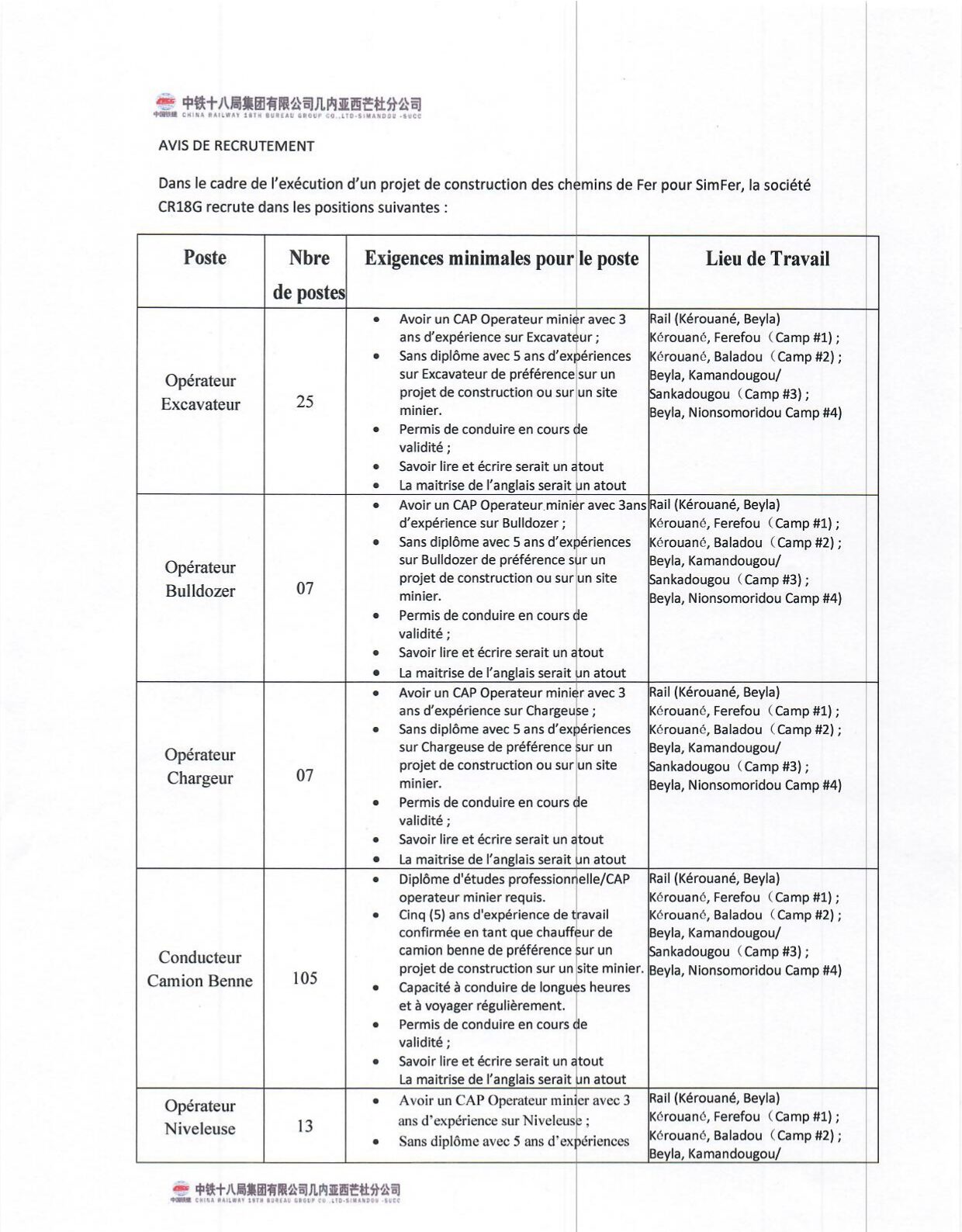
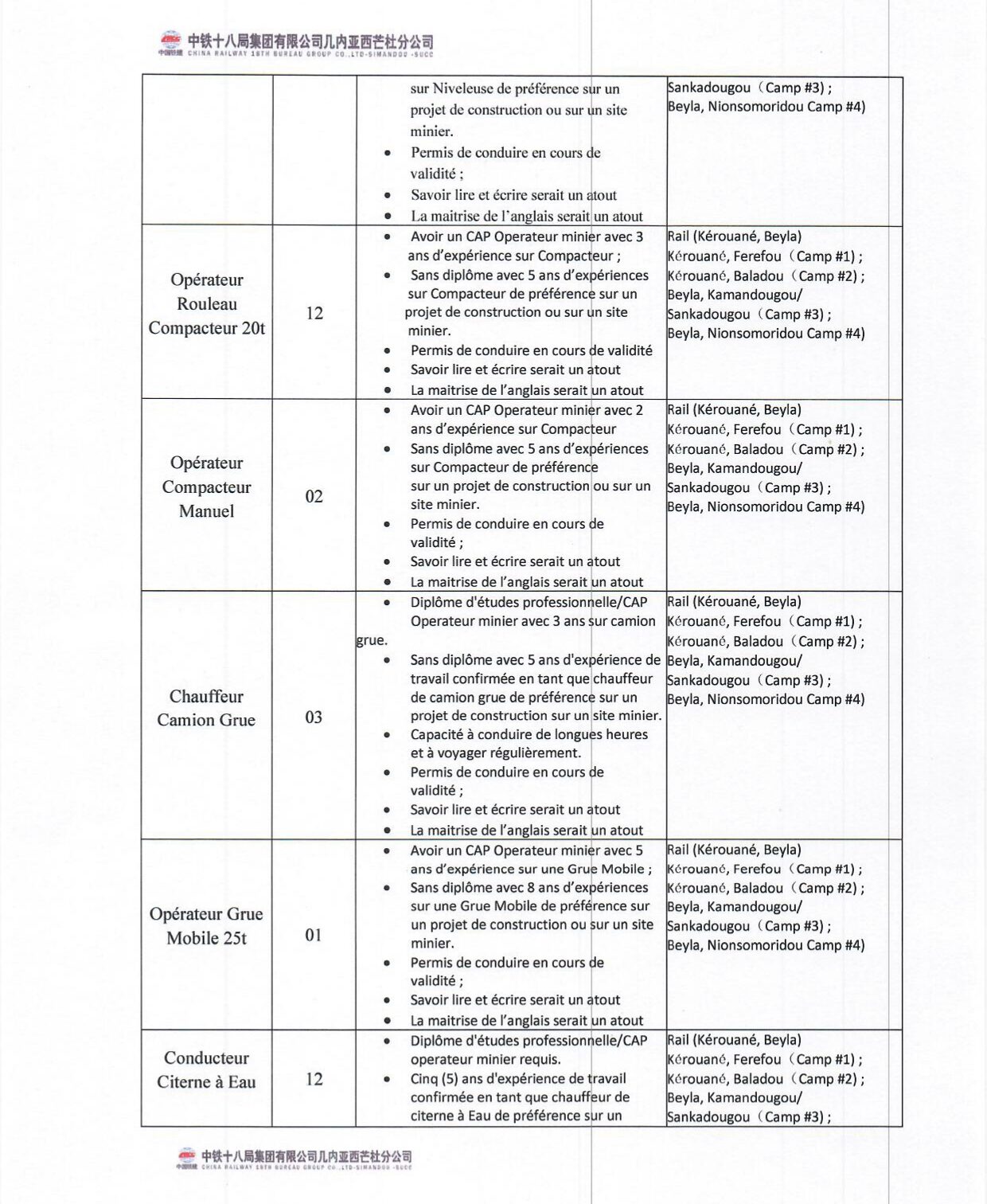
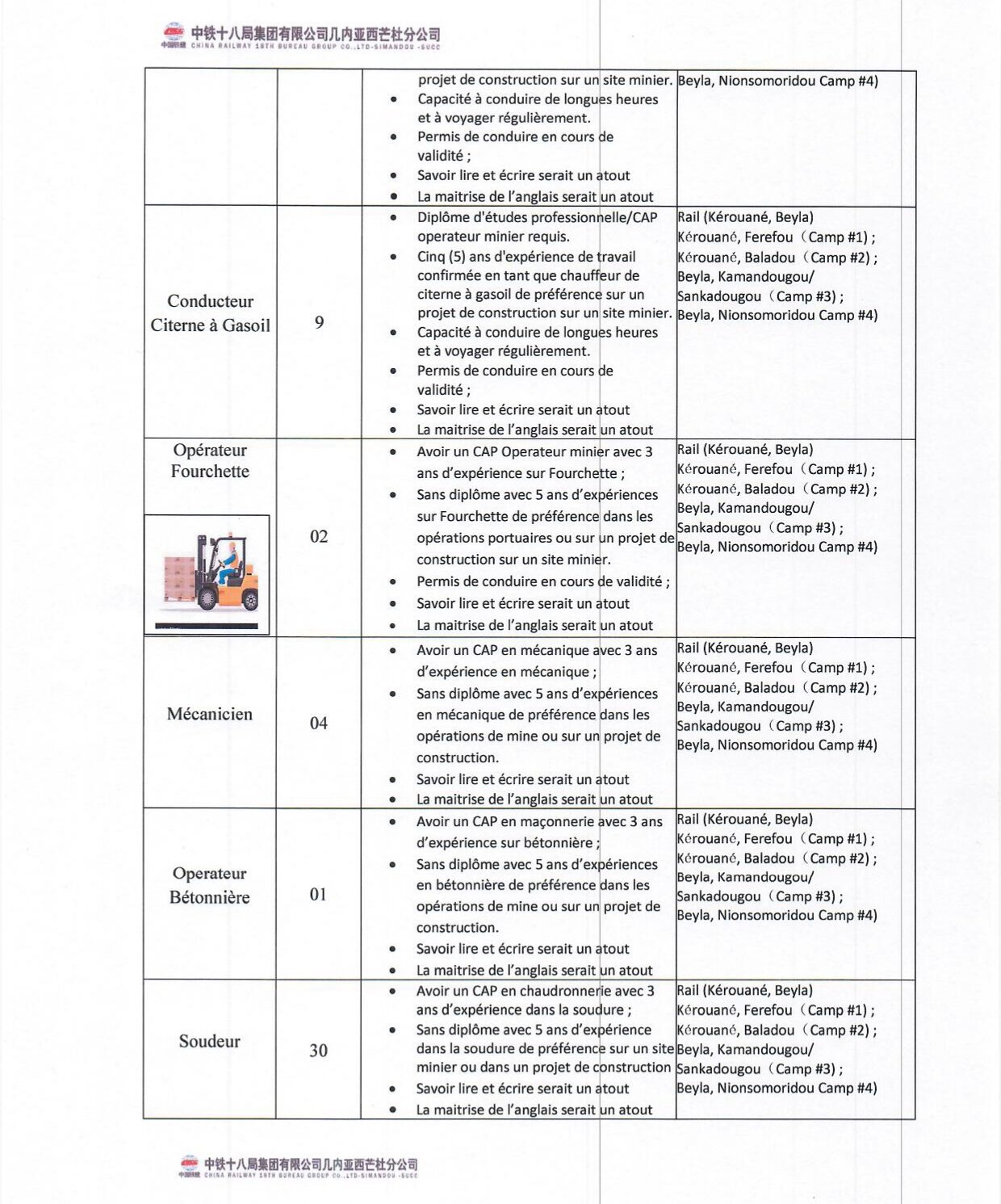
Ministère de la Santé et de l’hygiène Publique : PLAN DE PASSATION DES MARCHES 2024
| Autorité contractante : | Ministère de la Santé et de l’hygiène Publique | ||||
| Exercice budgétaire : | 2024 | ||||
| Ordonnateur : | Ministre de la Santé et de l’hygiène Publique | ||||
| Journaux de publication de référence et site Internet : | 1- JAO, 2- Horoya, 3- la République Site: ARMP/MSHP | ||||
| Autorité approbatrice : | DGCMP | ||||
Cliquez pour télécharger le TDR Projet-de-PLan-de-passation-2024-VF-valide_DGCM
MPTEN – RECRUTEMENT D’UN CABINET RELATIF AUX ETUDES DE FAISABILITE POUR LA CONCEPTION ET LA MISE EN ŒUVRE D’UNE NORME D’ADRESSAGE ET D’UN SYSTEME DE CODES POSTAUX EN GUINEE
REPUBLIQUE DE GUINEE
Travail – Justice – Solidarité
———————
Ministère des Postes, des Télécommunications et de l’Économie Numérique
———————–
Unité de Gestion du Projet
Projet de Transformation Numérique pour l’Afrique / Programme Régional d’Intégration Numérique de l’Afrique de l’Ouest (DTfA/ WARDIP).
SOLLICITATION DE MANIFESTATIONS D’INTERET
RECRUTEMENT D’UN CABINET RELATIF AUX ETUDES DE FAISABILITE POUR LA CONCEPTION ET LA MISE EN ŒUVRE D’UNE NORME D’ADRESSAGE ET D’UN SYSTEME DE CODES POSTAUX EN GUINEE
Date de début : 22 / Janvier / 2024 ; Date limite : 22 / Février / 2024
- Le Gouvernement de la République de Guinée a reçu un financement de l’Association internationale de développement (IDA) dans le cadre du Projet Transformation Numérique pour l’Afrique / Programme Régional d’Intégration Numérique de l’Afrique de l’Ouest (DTfA/ WARDIP), et à l’intention d’utiliser une partie du montant de ce don pour effectuer les paiements au titre du contrat suivant : Etudes de Faisabilité pour la Conception et la Mise en Œuvre d’une Norme d’Adressage et d’un Systeme de Codes Postaux en Guinée
Cliquez pour télécharger le document complet AMI-ADRESSAGE-WARDIP-7
FHI 360 recrute Senior Diagnostic Specialist
Internal and External vacancy announcement
Senior Diagnostic Specialist
Job Code: TECH 31019
Job Family: Technical
Compensation Band: LL
FLSA: Exempt
FHI 360 is a nonprofit human development organization dedicated to improving lives in lasting ways by advancing integrated, locally driven solutions. Our staff includes experts in Health, Education, Nutrition, Environment, Economic Development, Civil Society, Gender, Youth, Research, and Technology; creating a unique mix of capabilities to address today’s interrelated development challenges. FHI 360 serves more than 60 countries, all 50 U.S. states, and all U.S. territories.
Overview: The Senior Diagnostic Specialist will lead the diagnostics systems capacity-building work for the USAID Epidemic Control Project (EpiC) project in Guinea. EPIC serves the Global Health Security programs and other related initiatives. EPIC supports the Guinea government and relevant ministries in the strengthening of the Guinea human and animal health diagnostic systems for infectious diseases, and antimicrobial resistance (AMR). The work will focus primarily on public health systems but may also include private health and other non-governmental diagnostic facilities (laboratories and point-of-care facilities). The position will include in-country travel to diagnostic facilities and for diagnostic capacity-building activities and meetings. Technical responsibilities include working with the EPIC Guinea Team Lead, FHI 360 EpiC team, USAID, and local and regional EPIC Diagnostic Specialists to implement agreed strategies to enhance diagnostic networks (both human and animal health and point-of-care facilities) in Guinea as well as broader surveillance activities.
The key role of the Senior Diagnostic Specialist is to provide timely technical assistance to the National Institute of Public Health (INSP), the Direction National des Laboratoires (DNL), the Human, and Animal Health Reference laboratories; as well as the regional laboratories to further build their technical capacity to serve as the leader of the diagnostics network in Guinea.
Technical Responsibilities:
EPHI Responsibilities
The Senior Diagnostic Specialist provides technical assistance to the INSP to enhance or develop comprehensive systems at the national level and supports the INSP and DNL in adapting these to the regional and/or point-of-care level. Technical assistance will be provided in the following areas:
- Establish or upgrade diagnostic testing systems and SOPs for the detection of infectious diseases and antimicrobial resistance (AMR) with reference to international requirements.
This will include:
-
- Specimen acceptance and rejection criteria procedures
- Specimen preparation and transportation procedures
- Analytic procedures
- Quality control and quality assurance systems with feedback mechanisms
- Systems for recording and reporting results (individually and collectively into the national data management center at INSP
- Source documentation systems
- Archiving and repository systems for specimens and data
- Mechanisms for management review of diagnostic system performance
- Enhance equipment management systems including providing technical assistance to finalize equipment-related national documents such as the “Medical Device Management Policy” and advocate for the submission and approval; equipment (or diagnostic methods) selection and qualification procedures; equipment’ use procedures; routine preventive maintenance; troubleshooting procedures; servicing and repair systems; and backup systems for equipment failure.
- Enhance diagnostic commodities management systems including selection and quality testing of commodities, inventory management, procurement, and forecasting systems.
- Enhance systems for checking the quality of diagnostic testing through the use and timely review of internal quality control results, external quality assurance scheme results, proficiency testing program results, and other sources of quality indicator data.
- Enhance INSP quality oversight systems including review of quality indicator data (test turnaround time, QC/EQA data, testing downtime and cause[s], etc.), management review, and internal audits.
- Enhance human resource systems through the development or revision of standardized job descriptions for diagnostic staff and associated orientation programs.
- Establish systems for the training, competency, performance measurement, continuing education, and qualification of diagnostic testing staff and management.
- Enhance workforce resourcing and scheduling by monitoring the workload of functional areas with reference to testing volumes over peak and slack periods and proposing operational or staffing adjustments.
- Enhances laboratory information systems (LIS) by identifying information needs (user requirements) and problems, recommending improvements setting activities, responsibilities and timelines and assisting with the testing of new or refined systems (operational qualification).
- As part of this support the development of LIS use manuals and training of users.
- Enhances dialogue and the interface with the animal health sector for one health.
Country Responsibilities
EPIC will implement a suite of activities to build diagnostic capacity in Guinea. In addition to the technical support provided to INSP and DNL, EPIC will hire one full-time Diagnostics Specialist (DS) to work at the and sub-national levels to support QMS enhancements through the implementation of SLMTA.
The Senior diagnostic specialist will oversee the DS and provide technical oversight to ensure that national strategies and systems developed are translated into sub-national-level diagnostic systems and solutions. County-level activities for DS will include capacity building of diagnostic facilities and the network, quality improvement initiatives, strengthening of systems for supply chain and equipment management, and strengthening capacity for detection of pathogens and AMR.
Experience:
- A minimum of an MSc in clinical diagnostics.
- At least five years of experience in diagnostics implementation (on-the-bench experience) and management in Guinea or similar developing country settings.
- Proven knowledge and experience in the diagnosis of infectious diseases (including TB) and anti-microbial resistance including specific hands-on clinical diagnostics experience.
- Competent in current developments in the diagnosis of infectious diseases including point-of-care and molecular methods.
- Experience with implementation of laboratory quality standards including against ISO 15189 and/or SLMTA/SLIPTA or in-country standards.
- A demonstrated ability to work with multiple partners on collaborative projects.
- Experience implementing diagnostic network capacity building.
- Fluency in written and spoken French. Fluency in spoken and written English will be highly desirable.
Supervision Given/Received:
Will supervise at least one Diagnostic Specialist
Travel Requirements:
Some country-level travel
This job description summarizes the main duties of the job. It neither prescribes nor restricts the exact tasks that may be assigned to carry out these duties. This document should not be construed in any way to represent a contract of employment. Management reserves the right to review and revise this document at any time.
HOW TO APPLY:
Interested and qualified candidates are encouraged to apply by sending their application & updated CV through guinearecruitment@fhi360.org before/on February 02/2023
FHI 360 is an equal opportunity and affirmative action employer. FHI 360 is an equal employment and affirmative action employer whereby we do not engage in practices that discriminate against any person employed or seeking employment based on race, color, religion, sex, sexual orientation, gender identity, national or ethnic origin, age, marital status, disability, veteran status, genetic information or any other status or characteristic protected under applicable law.
Please make sure you write the position title you are applying for in the subject line. Failure to do so will lead to application disqualify.
Kindly note that only shortlisted candidates will be contacted.
Abt Associates – AVIS DE RECRUTEMENT CONDUCTEURS DE VEHICULE
Abt Associates est une entreprise américaine d’assistance technique et de conseil qui gère le projet PMI Evolve financé par l’USAID. Le projet PMI Evolve soutient l’initiative présidentielle américaine de lutte contre le paludisme (PMI) et l’USAID pour planifier et mettre en œuvre une approche intégrée de lutte antivectorielle dans le but global de réduire le fardeau du paludisme. A travers le projet PMI EVOLVE, Abt met en œuvre le programme intégré de lutte antivectorielle en s’associant avec les communautés locales, les organisations et les gouvernements. L’objectif final est de lutter contre le paludisme tout en renforçant les capacités locales pour pérenniser les acquis du paludisme
PMI Evolve apporte un appui technique au Programme National de Lutte contre le Paludisme dans la surveillance entomologique des vecteurs du Paludisme dans 16 préfectures de la Guinee et dans la zone spéciale de Conakry.
Pour le compte du projet PMI EVOLVE Guinée, Abt Associates recherche des candidatures qualifiées pour le poste ci-dessous :
Conducteurs de véhicule
https://egpy.fa.us2.oraclecloud.com/hcmUI/CandidateExperience/en/sites/JoinAbt/job/104760
Toutes les candidatures doivent être envoyées sur le lien ci-dessus en cliquant sur le bouton Apply Now avant le 31 janvier 2024
Conakry le 16 janvier 2024
Evelyne ALYKO
COP